By fusing together a pair of contorted molecular structures, Cornell researchers created a porous crystal that can uptake lithium-ion electrolytes and transport them smoothly via one-dimensional nanochannel, a design that could lead to safer solid-state lithium-ion batteries.
A paper on the work is published in the Journal of the American Chemical Society.
The project was led by Yu Zhong, assistant professor of materials science and engineering in Cornell Engineering and the paper’s senior author, whose lab specializes in synthesizing “soft” and nanoscale materials that can advance energy storage and sustainability technologies.
In conventional lithium-ion batteries, the ions are shuttled along via liquid electrolytes. But liquid electrolytes can form spiky dendrites between the battery’s anode and cathode, which short out the battery or, in rare cases, explode.
A solid-state battery would be safer, but that comes with its own challenges. Ions move slower through solids, because they face more resistance. Zhong wanted to design a new crystal that was porous enough that ions could move through some kind of pathway. That pathway would need to be smooth, with weak interactions between the lithium ions and the crystal, so the ions wouldn’t stick. And the crystal would need to hold enough ions to ensure a high ion concentration.
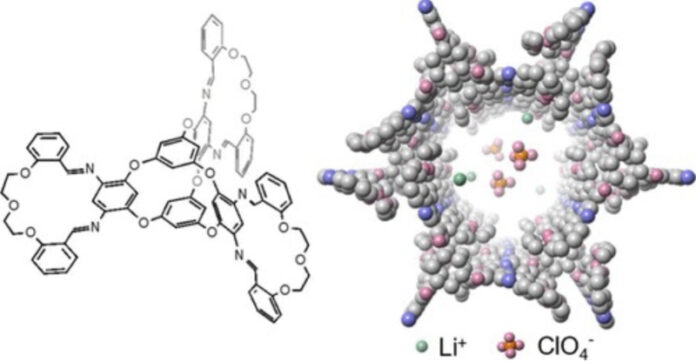
The team devised a method of fusing together two eccentric molecular structures that have complementary shapes: macrocycles and molecular cages. Macrocycles are molecules with rings of 12 or more atoms, and molecular cages are multi-ringed compounds that more or less resemble their name.
“Both macrocycles and molecular cages have intrinsic pores where ions can sit and pass through. By using them as the building blocks for porous crystals, the crystal would have large spaces to store ions and interconnected channels for ions to transport”, says Yuzhe Wang, lead author.
Wang fused the components together, with a molecular cage at the center and three macrocycles radially attached, like wings or arms. These macrocycle-cage molecules use hydrogen bonds and their interlocking shapes to self-assemble into larger, more complicated, three-dimensional crystals that are nanoporous, with one-dimensional channels—“the ideal pathway for the ion to transport,” according to Zhong—that achieve ionic conductivity of up to 8.3 × 10-4 siemens per centimeter.